What’s Inside
A partnership delivering for patients
Wet granulation, dry granulation, and direct compression are the most common processes for tablet manufacturing1. Realizing the potential for greater efficiency, quality and versatility, the pharmaceutical industry is increasingly looking to continuous direct compression manufacturing methods. The Medicines Manufacturing Innovation Centre initiated Grand Challenge 1 (GC1) to integrate Machine Learning and digital design tools to enable more efficient development and manufacturing of Oral Solid Dose (OSD) products. Applied Materials’ Pharmaceutical group has partnered with the Medicines Manufacturing Innovation Centre and technology partners Gericke and Siemens to deliver this Grand Challenge 1 project.
About CPI’s Medicines Manufacturing Innovation Centre partnership
The Medicines Manufacturing Innovation Centre, in Glasgow, has led GC1 in collaboration with the University of Strathclyde and founding industry partners, AstraZeneca and GSK. The main goal of the center, via a series of Grand Challenges, is to deliver on the accelerated development and integration of next generation manufacturing technologies.
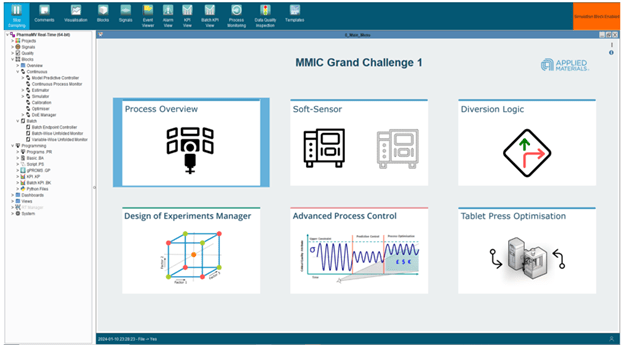
Grand Challenge 1 is one such endeavor within the Centre’s portfolio of projects. GC1’s objective is to further build on the efficiency of continuous processing by developing a digitally twinned continuous direct compression (CDC) platform and workflow. The platform provides flexibility from an equipment and digital perspective. The process line allows flexible configurations with support for feeders, blenders and tablet presses from different manufacturers. This physical flexibility is supported in the digital domain by technology partners including Applied Materials, Siemens and Gericke. Applied Materials has developed new automated workflows for Machine Learning utilizing our SmartFactory Rx, Smart Process Development solution. The solution, which is delivered using the PharmaMV application, integrates with a Siemens digital twin and SIPAT PAT Management system, while process automation is provided by Gericke line control. The digital platform provides model-based tools for characterization of powder properties, equipment, and process parameters, creating a good testing ground for the research and development into optimum processing conditions.
Integrated workflows reduce inconsistency and errors
Leveraging the Smart Process Development solution (see Figure 1), the Applied Materials’ Pharma group has focused on developing integrated workflows to help guide process development, optimization and Advanced Process Control development. Whereas typically the CDC production method requires experts in software, process development and manufacturing to develop a robust production solution, this innovative solution allows the line operator to execute pre-defined workflows, thereby simplifying the process. Among the benefits of these workflows is the reduction of inconsistency that arises when several experts work on the same task. Each is likely to do things a little differently, potentially leading to different outcomes. Another important benefit is that it enables pharmaceutical scientists to concentrate on their area of formulation expertise rather than focusing time and effort on the production solution.
These automated workflows bring advantages at many stages in the process of bringing a new drug to market.
Automated Experimental Design – Tablet Press Optimization
Balancing the edge thickness, weight and hardness of the tablet is critical, but also a challenge. Press parameters can vary significantly between each formulation as powder flow characteristics change with each API, excipient and lubricant mix. The formulation and process parameters must be optimized during process development. Further, the press must be set up by a skilled operator prior to each production run by applying a series of trial-and-error experiments in conjunction with compression tables and a tablet analyzer.
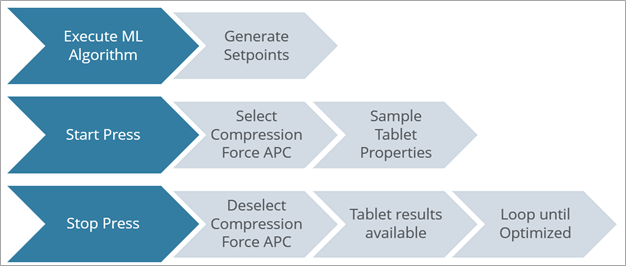
Applied Materials has demonstrated an alternative approach by providing a machine learning algorithm to automatically optimize the press. As shown in Figure 2, the workflow executes an initial set of experiments, then based on an optimization, determines the next best experiment to perform. This methodology efficiently determines the optimal startup configuration for dosing and compression forces to achieve the desired tablet weight, thickness and hardness.
This technique has significant potential to save material during the development stages, reduce waste as the process conditions are optimized and improve consistency during press set up. It can also be combined with Advanced Process Control to automatically control tablet properties in real time.
PAT/chemometric development
When a new drug moves into production, a new chemometric method is introduced into the line. Design of Experiments and calibration activities must then be carried out to generate a robust Quality Attribute prediction. Generating chemometric calibration models can be time consuming as different sources of information (spectra, calibration sample values, process conditions) are fused together. The usual workflow is to design the experiment, run one at a time, take an offline sample, test it, import data into spectra handling software, and potentially include additional process parameters that might affect the spectra. An experienced chemometrician is usually needed to perform the modeling in what can be a lengthy process, given you need to take data from different sources, combine it and manipulate it effectively.
To address these challenges, we developed the PAT workflow. Siemens supplied the PAT management system and assisted with integration of PharmaMV with NIR instrumentation. The streamlined workflow generates statistically rich data for building chemometric models for inline PAT. The software and user-friendly dashboards provide the operator with a series of pre-built templates. It guides the operator through setting up the experiments and then automates their execution, while ensuring process data, spectra, and offline samples are time aligned.
Soft Sensor Development
The Smart Process Development soft sensor workflow automates testing and creation of Machine Learning models. The soft sensor uses the feeder mass-flow values and a model that describes the Residence Time Distribution (RTD) to predict the blend uniformity. Previously, a series of step tests or tracer tests were conducted and then the RTD model was parameterized with this data. This is a time-consuming process, carried out post event, requiring custom scripting and extensive data fusion activities. It could also not account for any real-time changes in powder residence time. By leveraging reinforcement learning and control engineering techniques, the RTD model can be updated in real time. This workflow automatically produces an updated model that represents the process behavior, in just 10 or 15 minutes, and all at the press of a button.
Introducing the soft sensor offers the opportunity to introduce redundancy and increased robustness into the estimate of quality attributes. During routine manufacturing, the NIR and soft sensor methods provide two parallel predictions of the same attribute. The soft sensor provides a redundant backup for situations when the NIR is temporarily unavailable (due to drift correction or internal calibration etc.) Combined with Multi-variate Analysis, this redundant strategy also helps improve the robustness and tolerance of the system by negating sensor drift, model drift and sample presentation issues.
Diversion logic
Real-time diversion based on the soft sensor or chemometric method is now a well-established approach in continuous manufacturing. The concept is relatively straightforward; one or more Critical Quality Attributes are predicted in real time and then compared to the associated statistical limits. Should the Quality Attribute exceed the limits, then the tablet press diverter is activated, sending the tablets to waste.
The reality of achieving real-time diversion is considerably more complicated. Any diversion strategy must ensure robust diversion of out-of-specification product whilst minimizing yield losses from sending in-specification product to waste. This must be achieved while bearing in mind process dynamics, divert valve response times, data quality, NIR and feeder behavior, among other considerations.
The workflow streamlines the deployment and validation of diversion logic. PAT-Chemometric and/or soft sensor predictions can be combined. Guidance for threshold adjustment is provided based on measurement, process and diverter response times. Additional checks are also included to monitor data quality, PAT drift and communications connections. The workflow can be connected to the digital twin and data streaming tools to enable efficient validation of all aspects of the diversion logic. Finally, standard statistics can be selected and fed into Continued Process Verification tools and reports.
By using predictive diversion and making future forecasts, manufacturers can step from building models to being able to use predictions in those models to divert product to waste or to keep it. This workflow could be used to efficiently introduce new products into a line and increase the efficiency of the process overall. This approach also supports real-time release strategies in the future.
Conclusion
The Medicines Manufacturing Innovation Centre partnership, and particularly the work of Grand Challenge 1, will allow scientists to understand and optimize their formulation and process development for Continuous Direct Compression in the digital space. It also will reduce the amount of starting materials needed for optimization and the overall cost of moving from drug formulation, through process development into production for the end user. The dashboard workflows developed with our partners through GC1 enable increased efficiency in process operation. They have also introduced plug and configure tools for offline, process and PAT data fusion, Machine Learning for reduced experimental effort and advanced process control of multiple quality attributes.