Low (and variable) yield is an issue for API production
Even though most API manufacturing processes have been proven in use for decades, many companies experience low yield due to unforeseen (and at times unidentifiable) variability. This causes a significant impact on target production goals and sometimes requires costly re-work of batches (when possible) and additional safety stock.
This effect is compounded by the globalized nature of API manufacturing, with supply chain disruptions that can lead to delays, inefficiencies, and increased costs of API production. The unpredictability of the supply chain requires a risk management strategy to ensure the continuity of operations. This is further exacerbated when production is not running at peak performance, resulting in costly material waste.
These challenges become even more daunting for global pharmaceutical manufacturers that operate numerous production lines and facilities across the globe, sometimes using the same equipment to process and produce multiple products. Many manufacturers are continually focused on ways in which yield can be increased and variability reduced in API manufacturing as a way of improving supply chain reliability.
Identifying the root causes behind yield issues
With an understanding of the constraints and challenges, let’s look at how one pharma manufacturer was able to embrace and harness the power of digital transformation to optimize their global operations. Initially, they identified three root causes behind process yield issues: (1) a lack of real-time visibility into critical process and quality parameters within in-process steps, (2) delays in reacting to production issues, and (3) the absence of a systematic approach to predict quality, creating a reactive approach to resolving issues.
The customer was taking a series of critical quality measurements during several reaction phases to understand the conditions of the batch being manufactured versus regulated limits. However, they were not using this information to understand the general health (and yield) of a batch. If the API being produced was not meeting the required critical quality attributes, the customer had to manually calculate and add or dilute API raw materials to bring the production process back within quality specifications – or in some cases abort the batch. This in turn impacted supply chain predictions of raw material consumption. It also caused lower yields with no systemic understanding of what was causing the batch-to-batch variability. This resulted in significant increases in re-work (reprocessing times of up to 12 hours), missed production schedules, and lower Overall Equipment Efficiency (OEE).
The pharma manufacturer determined that the best way to improve these key metrics was to implement an analytics system and a digital transformation of their plant to better identify the drivers of yield-impacting problems. By identifying these problems earlier, they aimed to make informed decisions quickly to minimize yield drift and raise overall performance.
Driving better yield using an analytics-first approach
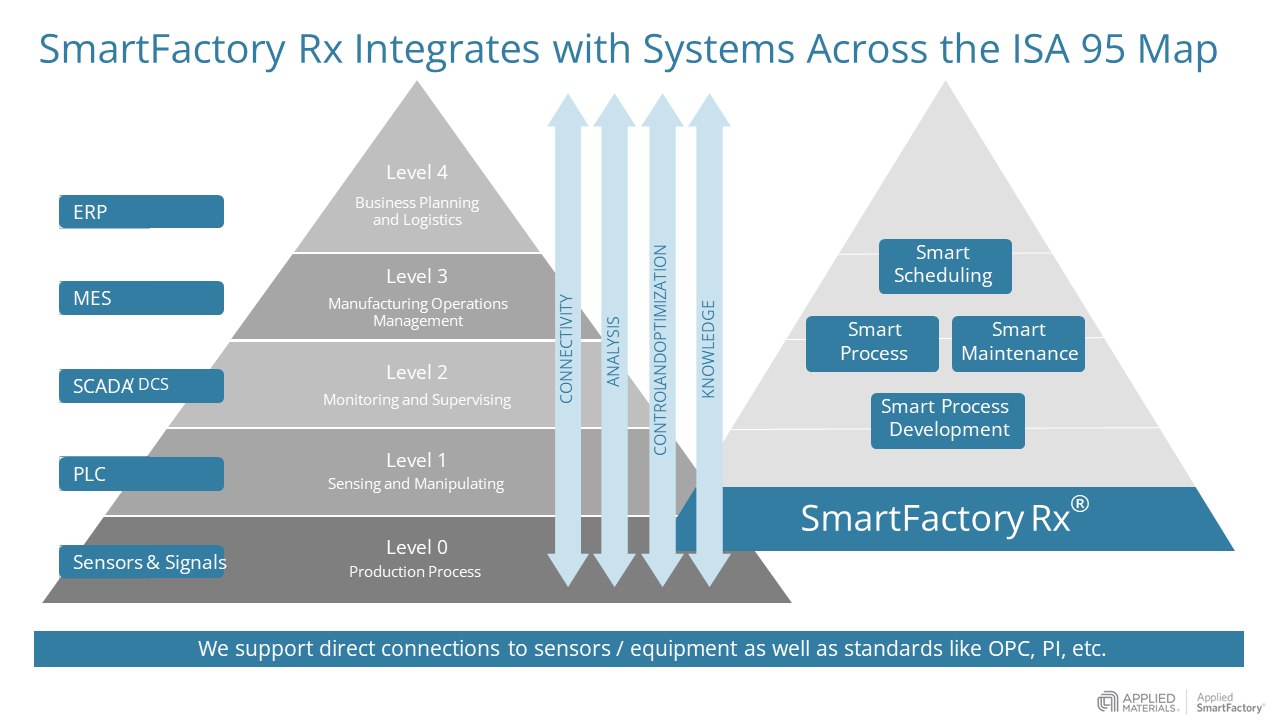
The pharmaceutical manufacturer implemented Applied Materials’ SmartFactory Rx® solution suite to help identify the root causes of yield loss and variability using an automated data driven approach. The SmartFactory Rx team worked side-by-side with the customer to conduct a series of Gemba Walks to perform root cause identification of the manufacturing process issues. By focusing on the key unit operations – and associated critical process and quality parameters – the team developed a solution plan to implement real-time quality control, optimized process monitoring, and predictive alerts to drive higher yield. These tools built on a framework of pre-built systems integrations, allowing the team to plug into manufacturing execution systems (MES) as well as PI and OPC to obtain both historical and real-time data to analyze and model the process.
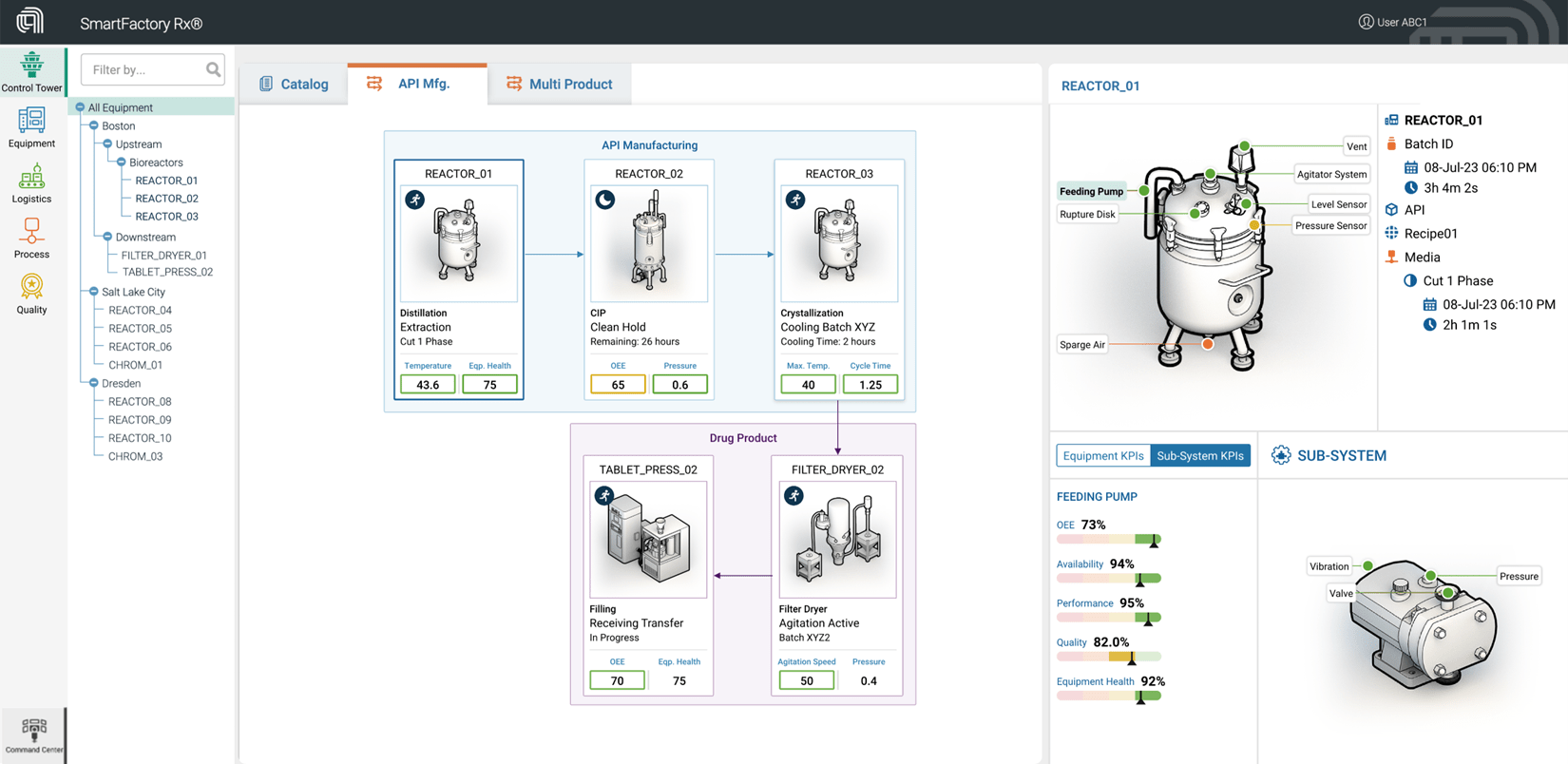
This ‘analytics-first’ approach allowed operators on the shop floor to directly see, track, analyze, and optimize the entire process thanks in part to SmartFactory Rx’s ability to seamlessly integrate into the customers data eco-system (see Figure 1). By providing data-driven transparency in end-to-end operations, they allowed technicians to make critical decisions in near real-time for a single production line. Learnings at individual sites were then compared to similar API sites across the enterprise. Key statistical models and methods of analytics were then deployed to other production facilities to facilitate continuous improvement initiatives. To ensure repeatable outcomes, the SmartFactory Rx team also aided the development of best practice work instructions that culminated in significant annual cost savings in the millions of dollars for the client.Using this approach enabled teams to focus not just on operating within the validated process range, but to identify how patterns in behavior affected critical attributes. Machine learning and other data analysis and modeling techniques were then deployed online so that operators could easily monitor and see intelligent, but easy to understand, information in a simple ‘control tower’ view of their facility (see Figure 2). Furthermore, SmartFactory Rx was able to offer alerts along with prescriptive actions when a trend demonstrated non-optimal processing conditions. The operators on the production floor would receive visual guidance and warnings via the online interface as well as guidance on when (and sometimes how quickly) to perform certain actions. Supervisors would receive email updates for transparency.
The results were striking. There was a significant reduction in overall cycle time and raw material waste and a 7% improvement in yield on average across batches. This resulted in millions of dollars in savings annually. The manufacturer also saw significant changes in operational consistency. This was driven by giving operators direct access to prescriptive action alerts and with step-by-step instructions on what to do when operational limits or critical quality attributes were driving towards negative trends. The client was able to identify and correct deviations in near real-time, resulting in a 25% increase in manufacturing productivity, better equipment utilization, and improved OEE.