As a patient, when thinking about a pharmaceutical manufacturing operation, what comes to mind—cleanliness, purity, high levels of control, high quality? The truth probably involves all these measures and many more. Pharmaceutical manufacturing carefully tracks many critical quality attributes (CQAs) for specific products and monitors how those quality attributes relate to the process parameters that are used to make them. This crucial link between process parameters and quality attributes is essential to understanding the process and to ensuring the manufacturing process is continually monitored, assessed and kept under a tight state of control. While this type of analysis has been done for compliance reasons for decades, new analytics tools are helping manufacturers leverage this data to drive real changes on the shop floor.
Keeping the process in control
Limitations of manual data treatment
It is still common for CPV approaches to be manual and non-automated. Data Science teams will manually query, process, and analyze data; such a laborious approach can lead to introducing manual transcription errors, missing data and a range of inconsistencies. It’s also slow and cumbersome to collect this data, since it’s usually in a variety of systems (e.g., quality results in Laboratory Information Management Systems [LIMS], process data in Manufacturing Execution Systems [MES] and other sources).
Due to the time and data synthesis requirements, analysis tends to be periodic and one-dimensional in nature. Information is rarely shared between teams working on the same product/line, which may result in disconnected data and lost opportunities for improvement.
To overcome these shortfalls without increasing workload or incurring extra data processing burdens, we need to think differently. We need to think about our data as an enterprise-wide real-time asset that can help drive true improvement in processes, not locked in spreadsheets that are inaccessible to users. The ability to streamline these sources of data into a single, common platform has the potential to address many of the hurdles that could be encountered. [2]
Continuous improvement Process Verification (CiPV)
SmartFactory Rx is designed as a Continuous improvement Process Verification (CiPV) platform that implements and builds on traditional CPV-type analytics with a set of tools that focuses on solving the collection, extraction and reporting process to allow more frequent monitoring and reporting.
Such an approach allows teams to automate much of the process of collecting and synthesizing the necessary data, thereby allowing a real-time approach to process improvement. This allows the unification of data across multiple data hierarchies, bringing the ability to trend outside of the usual CQAs which can facilitate advanced modelling outputs, and root cause analysis.
The SmartFactory Rx GxP-compliant data pipelines provide a carefully controlled way to ensure consistent handling of data. This provides verified ways to merge data across multiple data sources (e.g. LIMS, MES, historians). It is breaking down these data silos that removes one of the main barriers to producing high quality analytics.
The real-time, automated data pipeline also ensures that data is current, meaning there is a single source of real-time information for quality-based analytics. By reducing data latency, we can reduce delays in crucial decision making which might otherwise result in quality control issues, increased costs, or delays in the production of critical, often time-sensitive medicines.
Built for compliance
SmartFactory Rx is built to support harmonization with ICH guidelines for the efficient, safe development of much needed medicines on a global scale. In particular, CiPV was designed to support QbD (Quality-by-Design) and ICH Q9 – Quality Risk Management guidelines. Both of these methodologies require a data-driven approach that looks at process capability within established, empirically tested designs-of-experiments during process development.
The tool’s Quality Domain allows the user to monitor and compare quality parameters across the enterprise, giving insight into root cause, improving yield and supporting real-time release and product diversion strategies.
Reporting improvement
Sharing both inputs and outputs is beneficial across organizations looking to maximize learnings from their data. Using a platform with a design-led interface, users can easily create bespoke analyses and tailor reports to share among their team and the wider organizational network. By accessing data from across the entire process to build traditional univariate and multivariate models and harnessing AI/ML, confidence predictions and quality-based analytics are readily analyzed and visualized to deliver the results of CQAs, CPPs and Key Performance Indicators (KPIs).
These real-time reports are currently used to drive tangible improvements on the shop floor. For example, in manufacturing handover meetings each morning, teams view the current performance trends for active batches around manufacturing stability and performance. They are used to identify out-of-trend, drift and other pattern-based analytics to enable early intervention in the case of a process that is not performing well, or to indicate where a process won’t be performing well in the future. These findings can be implemented in simple ways that allow teams to take actionable steps, early, as well as to enhance overall manufacturing practices. This provides particularly valuable information in start-up for example, where there may not be a long history of steady-state performance data for a particular piece of equipment on which to base decisions.
Models for change
More advanced, multivariate models can also be used to provide additional sensitivity to pick up drift and change in performance prior to univariate responses. However, these models can also be hard to interpret. Within SmartFactory Rx, the main contributors to such deviance are clearly shown and they can also be trended and investigated. Furthermore, where appropriate, the raw data, e.g. process temperature trace, can also be viewed. This seamless workflow results in faster time to diagnosis and therefore to remedy.
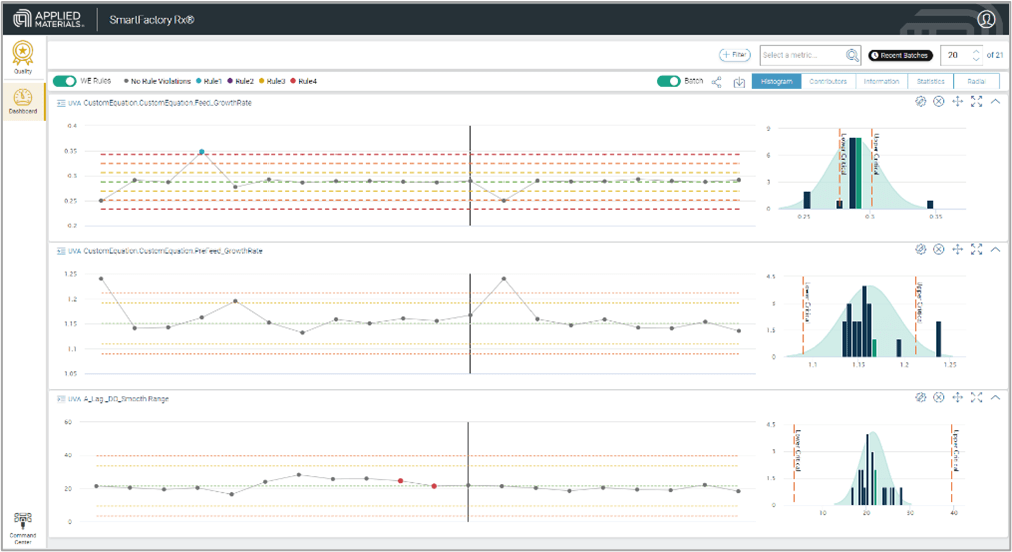
Identification of deviation from expected behavior can easily be spotted in the Quality Domain of SmartFactory Rx, as shown in figure 1, below. Traditional rule-based highlighting of points is deployed, using Western Electric logic. These rules, as well as the specification of custom limits—including limits that change over time based on for example, performance, audits or CAPA related enhancements—enable a more comprehensive, continuous improvement approach to quality. The platform can also be configured to automate notifications and workflows to address deviations or to trigger cadence-based reviews rather than relying on manually triggered workflows.
Further to traditional quality monitoring, these approaches should be adopted by manufacturing to identify areas of improvement, process drift and other key performance indicators. The sharing of data and methods through a common platform compliments a multi-team, multi-discipline approach to pharmaceutical manufacture, or in other words, helps break the silos that exist within complex, regulated manufacturing environments.
Balancing risk
The ability to provide continuous monitoring and reporting of CPPs and CQAs (including SPC monitoring and process capability measures, as well as more advanced tools) helps ensure quality, drive continual improvements to throughput and can also support real-time assurance for product release. For example, methods can be implemented to tackle the challenge of managing out-of-spec product. By utilizing a Residence-Time Distribution (RTD) strategy, targeted diversion of out-of-spec material can be implemented. Using a dynamic model, e.g. [3], to predict quality attributes and using this as part of the control strategy, leads to an optimized balance between risk of product quality and cost of losing in-spec material. Utilizing continuous monitoring and reporting approaches and functions can vastly speed up informed decision making.
The related statistical reports add to the story and allow the user to confirm their visual observations as well as give further metrics on process capability. These are automatically generated and are simple and easy to use.
The same approach can be leveraged for the analysis of machine learning (ML) strategies and their performance. The drivers of yield or any other CQA can be revealed, and their relative importance trended. Changes to these algorithms based on behavior, as well as identifying more optimal settings for the parameters underlying the ML models, can then help drive continuous improvement to these models. So, a common approach can be used to improve process performance as well as our ML based digital twins.
In summary
[1] https://www.europeanpharmaceuticalreview.com/article/29903/continued-process-verification-a-challenge-for-the-pharmaceutical-industry/
[2] https://www.pharmamanufacturing.com/compliance/regulatory-guidance/article/11288316/supporting-continued-process-verification
[3] Garcia et al. (2017), AiChE Journal,
https://doi.org/10.1002/aic.15967