Personalized medicines are poised to revolutionize the healthcare industry by tailoring medical treatments to the individual characteristics of each patient. They promise to provide more effective treatments that are precisely targeted to a disease, using revolutionary new genomic approaches like CAR-T. According to a report by Grand View Research, the global personalized medicine market size was valued at USD $538B in 2022 and is expected to grow at a compound annual growth rate of 7.2% from 2022 to 20281. There are nearly 50 products in phase 3 poised for approval, and a tidal wave of more than 250 products currently in phase 2 trials covering a broad range of indications from oncology to CNS, metabolic products, and more2.
However, drug manufacturers are struggling to efficiently manufacture individualized medicines at low cost and deliver them in a timely manner3 4. This issue is increasingly becoming a major issue for new drugs entering the market, as manufacturers seek to drive down ballooning manufacturing costs and manage supply chain variability and production delays. In this blog, we give an “operations research” perspective into the ways in which personalized medicines will require major changes to the tools, processes and thinking of pharmaceutical manufacturers.
The rise of personalized medicines
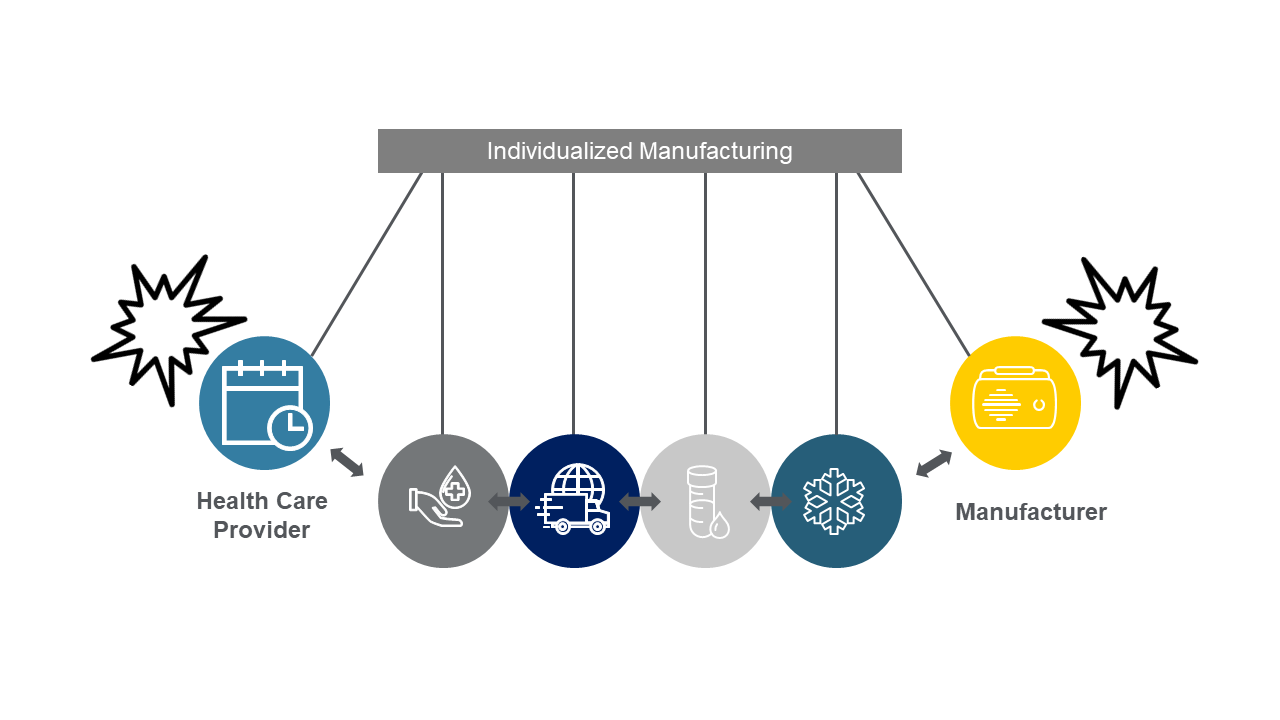
Personalized medicine is based on the understanding that each patient is unique, from their genetic makeup to their lifestyle and environment. Today, most approved personalized medicines – e.g., Breyanzi, Kymriah, Tacartus, Yescarta – as well as those in phase 3 (close to approval) use autologous cell therapy approaches that use a patient’s own cells as the starting material for treatment. Manufacturing begins with blood collection at the primary health care provider’s office (leukapheresis), followed by transportation to the manufacturer, production and QC testing and, finally, transfusion of the treated cells back into the patient.
This individualized manufacturing method is a major departure from traditional small- or large-molecule pharmaceutical manufacturing methods that are “made to stock.” In that case, one batch isn’t made for a specific patient but is produced well in advance of demand to treat hundreds or thousands of patients, often taking six months or longer to manufacture and release. In personalized medicine manufacturing, manufacturers must “make to order” each individual patient batch and require much shorter manufacturing and quality release lead times that are one month or less (since the treatments are life preserving). Delays in manufacturing for many personalized medicine therapies potentially reduce a specific patient’s chance of survival, further raising the stakes for manufacturers.
“Tight coupling” between manufacturers and primary care providers
More Batches, More Problems
Today, the manufacturing of drug substance (the raw material that is the active pharmaceutical ingredient in the drug) is made in large batches, each designed for many patients. A typical manufacturing facility will produce several hundred batches in a year; many biologics facilities (making more complex drugs that are the closest manufacturing analogue to autologous cell therapies) make less than a batch a week. Every part of the facility—from the automation system to materials handling systems, operator staffing, and even the design of rooms and the work instructions/SOPs managing tasks – is designed to make a small number of batches each day. In this paradigm, a supervisor or scheduler will often know where all the concurrent batches are being made in the facility at a particular moment in time.
Personalized medicine, in contrast, will require the just-in-time production of tens of thousands of patient doses a year – which will mean up to a thousand batches in production at a facility at any one time. In this new manufacturing modality, there is no way for supervisors to know where all the batches are; there’s just too many of them. Worse, IT systems that have been designed and scaled for a small number of batches don’t work well in this environment.
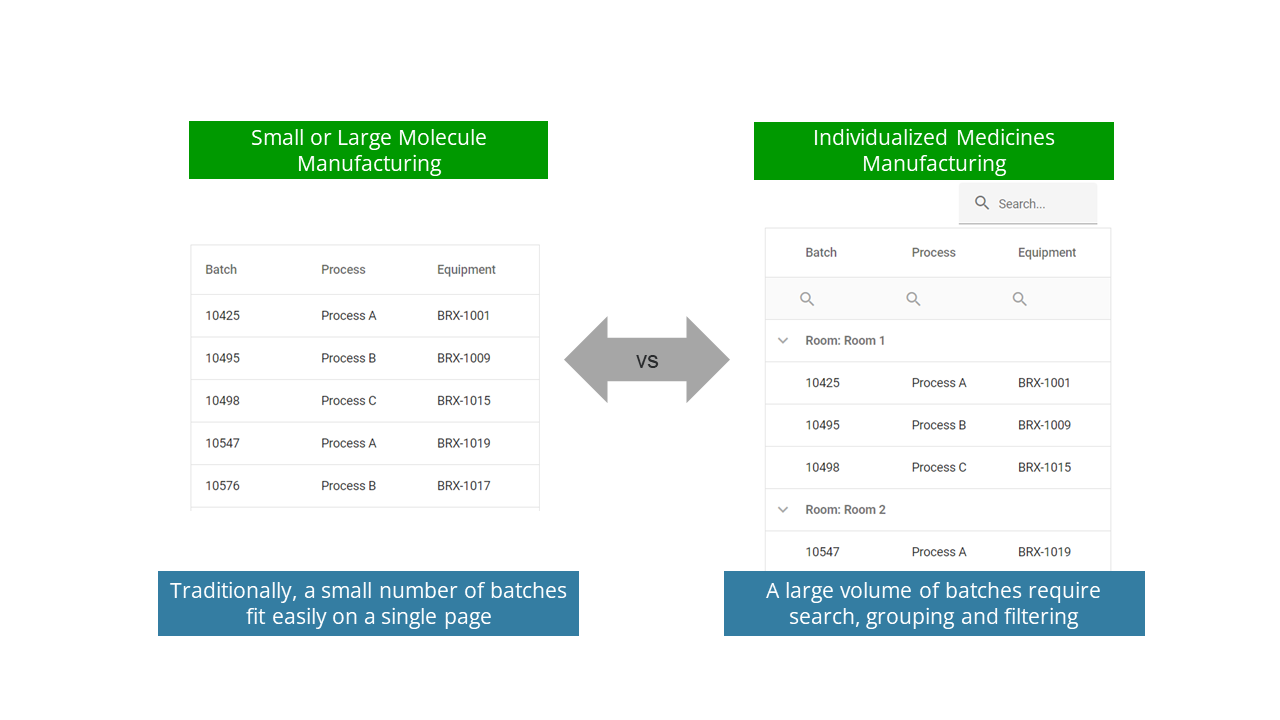
Consider the example in Figure 2 below. The first image is what we think of as the simplest possible example we’ve seen on the shop floor: a list of batches currently being produced on a manufacturing status board or on a manufacturing level display (HMI). In the left screen, we show the typical number of batches being made in a traditional facility. An operator has no need to scroll to see all the batches: they all fit on a single page.
Conversely, individualized medicines require methods to search, group and filter the data within the application since there is no way a user can see all the batches in production at the same time. This may require (for example) adding other metadata like the room in which production is occurring, to make the situation clearer to operators. As most traditional MES/DCS systems don’t incorporate this type of search, grouping and filtering, operators find them clunky and difficult to use for personalized medicines.
This may seem like a simple example, but it has powerful ramifications across the enterprise. Many automation software products have sorting or joining operations that perform very poorly with an increasing number of simultaneous production batches. Scheduling software and algorithms are a particularly important case since optimal scheduling for pharmaceutical manufacturing is known to be an NP-hard problem. Applying algorithms designed for small problem sizes will take a very long time (hours or days to solve), or will just not work, for individualized medicines manufacturing.
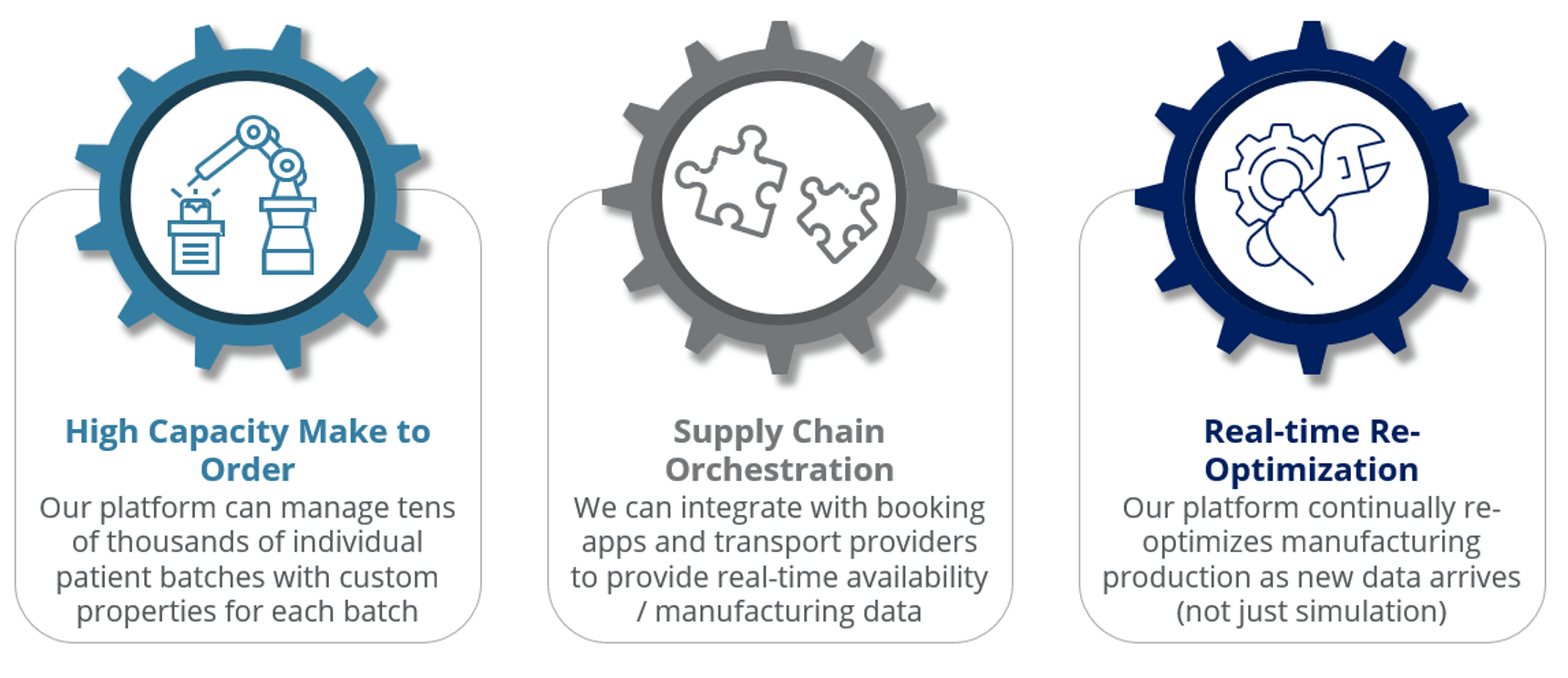
Applying lessons from the semiconductor industry
Conclusion
References
[1] Personalized Medicine Market Size, Share & Trends, available at: https://www.grandviewresearch.com/industry-analysis/personalized-medicine-market
[2] IQVIA Pipeline Intelligence September 2021; IQVIA MIDAS MAT Q4 2021; 2021 Company financial statements
[3] Eric Palmer / Fierce, “Novartis, others face higher manufacturing costs with CAR-T cell treatments”, available at https://www.fiercepharma.com/manufacturing/novartis-others-face-higher-manufacturing-costs-car-t-cell-treatments
[4] Genetic Engineering News, “Will Manufacturing Be the Stumbling Block for T-Cell Therapies?”, available at https://www.genengnews.com/insights/will-manufacturing-be-the-stumbling-block-for-t-cell-therapies/